As compared with single-photon microscopy, two-photon microscopy uses longer wavelength for excitation and enables high-resolution imaging in deep tissue. In conventional two-photon microscopy, a galvo mirror or a resonant scanner is used for point-by-point raster scanning, while the emission fluorescence is detected by point detectors such as photomultiplier tube (PMT). However, different regions of biological tissues have different and heterogeneous refractive indexes, which lead to different illumination light paths, aberrations in image formation, decrease signal-noise contrast and pose the ultimate depth limit to the two-photon microscopy. To improve the penetration depth, wavefront sensing and adaptive correction on excitation can be used in two-photon microscopy to achieve near theoretical resolution and acquire images of high contrast at deep tissues [Kai Wang et al, Nature Method 2014; Kai Wang, et al, Nature Communication, 2015].
However, two-photon microscopy still suffers from two disadvantages, a low axial resolution and a slow imaging speed due to the point-to-point excitation and image formation. In contrast, light-sheet microscopy confers fast three-dimension volumetric imaging with low photo-bleaching and photo-toxicity, at the expense of spatial resolution. Previously, our colleagues have developed a two-photon three-axis digitally scanning light-sheet microscope (2P3A-DSLM) that achieve a lateral resolution of 400 nm, an axial resolution of 800 nm within a volume of 200x200x200 mm3, along with reduction in photo bleaching to 1/10 of the two-photon point scanning microscope. The only problem, we believe, is the field-of-view (FOV) that is limited by the aberrations in excitation and emission paths.
To extend 2P3A-DSLM high-resolution imaging capacity in three-dimension, we want to combine the 2P3A-DSLM with adaptive optics.Because light-sheet microscopes detect emission fluorescence all at once via sCMOS/EMCCD cameras, we need to compensate for different aberrations originated from multiple isoplanatic regions simultaneously. To extend penetration depth, we propose to use dual-layer wavefront sensing and correction technique, which will use two wavefront sensors and two deformable mirrors working at pupil plane and back pupil plane, respectively. We also plan to compensate for aberrations in the excitation path, which has never been done previously and will extend the next generation of light-sheet microscopy in the world.
Adaptive Optics Based System design
As shown in Fig.1, the two-photon excitation femtosecond laser pulses pass the electro-optic modulatior (Conoptics), expanded by a 2X beam expansion (Thorlabs, BE02R/M), cut by an aperture stop, penetrating a TAG (TAG OPTICS), passing a 4f system, reflected by a 2-axis MEMS (Mirroracle), penetrating a scan lens (Thorlabs, LSM04-BB) and a tube lens before collimating on the rear face of excitation objective (Special Optics, 28.6X/0.7, water immersion). We will use the wavefront sensor and wavefront corrector to ensure the quality of excitation point in the specimen. In the emission system, we used an objective (Zeiss, 40x/1.0, water immersion) with the piezo scanner (Piezo system jena) to match the excitation objective. We will use the scan lens, two galvo scanners, a 4f system, a dichroic mirror to deliver the collimated femtosecond laser pulses (Coherent, 920/1060nm) to do the excitation with de-scanning. Passing through another 4f system, we will place a deformable mirror (Boston, DM140A-35-UP01) at the third conjugated plane of pupil, and a S-H sensor (Photometrics, EMCCD) at the forth pupil conjugated plane. In this way, the aberrations from different isoplanatic regions at different depths of the specimen could be measured by the de-scanning wavefront sensing method [Kai Wang et al, Nature Method 2014]. After switching the beam splitter into the path, we will place another deformable mirror (ALPAO, DM97-15) with large aperture located at the back pupil plane where the wavefronts from different isoplanatic regions spread overlapped over the plane. We will use another wavefront sensor (Photometrics, EMCCD) conjugated to back pupil plane to detect fluorescence signals and determine the relationship between two wavefronts distributions at the pupil plane and the back pupil plane. Then, we can use the second DM to correct for the composite aberrations from the whole FOV. By flipping the beam splitter into the sixth pupil plane, we use a sCMOS (Hamamatsu, Flash 4.0) camera to detect the compensated emission photons. All lenses in the emission system will have both visible and near infrared anti-reflective coating, and two single-axis translation stages will have with high repeatability in switching between two beam splitters.
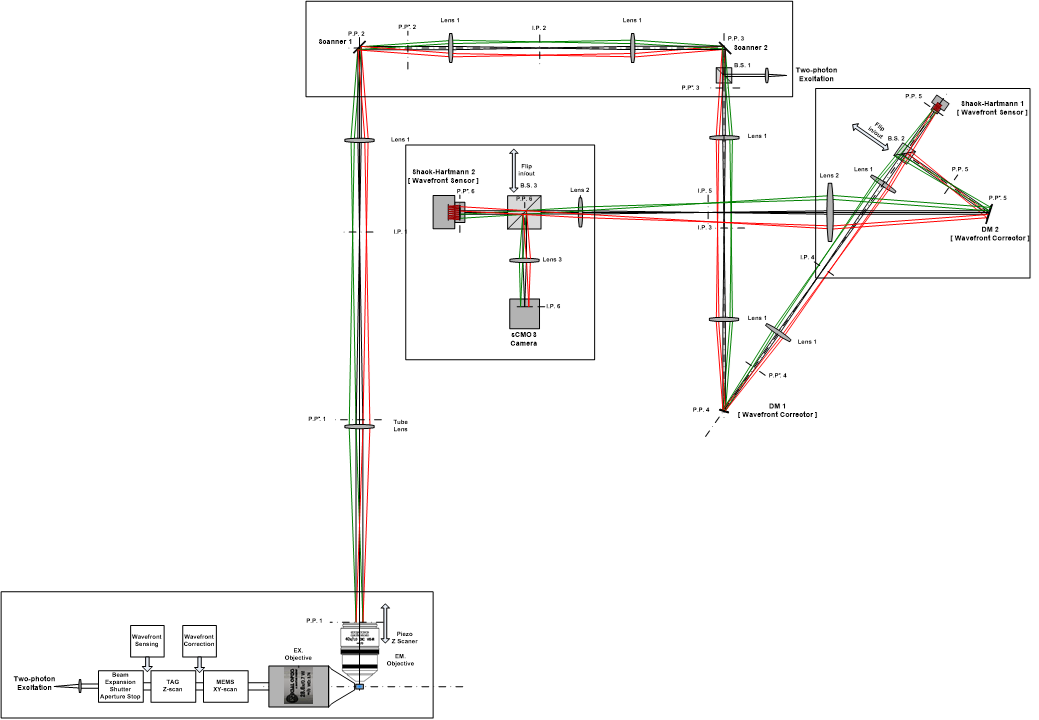
Fig.1 Schematic of the proposed two-photon light-sheet microscope.
Current works
[1] We currently have finished the implementation of excitation system and alignment of two objectives with high positioning accuracy, as shown in Fig.2.

(a) Excitation system on the table top

(b) alignment for two objectives
Fig.2 Layout of two-photon light-sheet microscope under construction.
[2] We are working on exploring the best optimization strategy to place the aberrations from different isoplanatic regions into a few sections on the back pupil plane, and to find the optimal position of back pupil plane basing on the in-situ system design.
[3] We are working on determining the parameters of microlens array on first S-H sensor.
[4] We are developing a FPGA Timing Control GUI, which is to enable real-time control of deformable mirrors via the serial port.
Grants
[1] Investigator, Super Spatio-temporal Resolution Miniaturized Microscopy for in vivo two-photon imaging, NSFC Major Scientific Instrument Development, 2014.01-2018.12
Publications
[1] Xiaoshuai Huang, Junchao Fan, Liujun Li, Haosen Liu, Runlong Wu, Yi Wu, Lisi Wei, Heng Mao, Peng Xi, Liqiang Tang, Yunfeng Zhang, Shan Tan, Liangyi Chen, Visualizing millisecond intermediates of vesicle fusion pore in live cells with ultrafast super-resolution microscopy, manuscript in preparation.
[2] Caihua Zhang, Zhiwei Zhao, Liangyi Chen, Zhaoliang Cao*, Heng Mao*, Application of adaptive optics in biological fluorescent microscopy, SCIENTIA SINICA: Physica, Mechanica and Astronomica, 47(1), 2017.
[3] Heng Mao*, Louis Tao, Liangyi Chen, Application and development of Adaptive Optics to three-dimensional in vivo deep tissue fluorescent microscopy, Infrared and Laser Engineering, 45(6), 2016.
Patents
[1] Heng Mao, Louis Tao, Ming Jiang, Liangyi Chen, Haiwen Li, Jie An, Weijian Zong, A High Spatio-temporal Resolution Light Sheet Fluorescence Microscope and Imaging Method, Invention, No.201510683873.1, application date Oct 20, 2015.